配体配合式检查最简单的技巧(LBA)是mAbs怪物浅析的大部分军,直接高效液相色谱-质谱最简单的技巧(LC-MS)也在近年来变的更越受喜欢。这二种浅析方法也大部分使用于免疫抗体药材和衍怪物的怪物浅析。
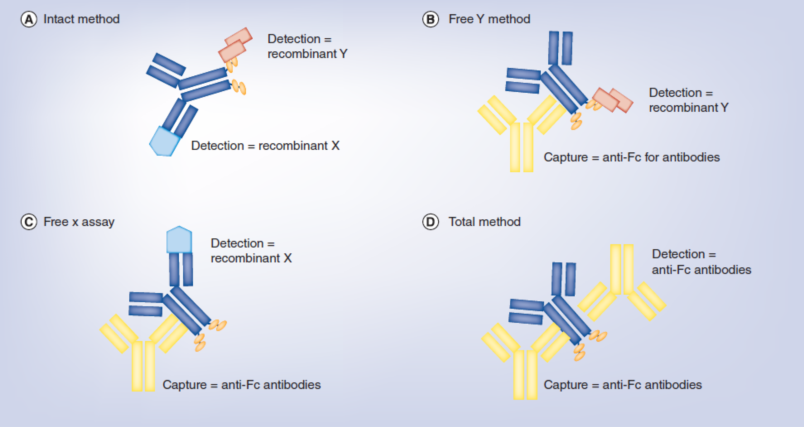
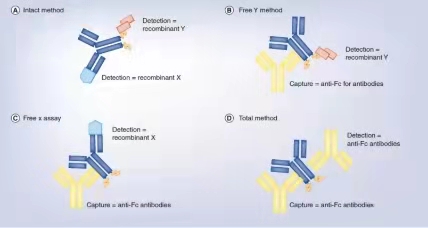
双特情人分子式不断增加了PK3D建模 的繁多性,举例子,表现靶点介导的制剂处治需求注重5个靶点的表示,因此以各种不一样的声音频率存在在各种不一样的受损细胞类形上。“分离”制剂氨水含量与“总”制剂氨水含量的降钙素原检测,就PK/PD3D建模 至关非常重要,特备是当牵涉可可溶性靶点时;仅是,基于靶标球蛋白在身体里的各种不一样表示摸式(expression patterns),制剂降钙素原检测探讨会会变的愈来愈繁多。在绝半数以上半数以上的医学设计中,制剂氨水含量是在并不是的“地方隔室central compartment”或设计不断循环(systemic circulation)中,以血浆或血清样例的行式,检验的,这在比较大数量上是基于方便从口服制剂的受试者中送样。会按照二十多年积攒的科学的数值文件,设计制剂氨水含量(systemic drug concentrations)表示了制剂在靶标能力步位的氨水含量。血渍病是个例外情况,但即是是血渍病也大环节内含无水硫酸铜团队的环节。设计制剂氨水含量和能力位置(site-of-action)的制剂氨水含量两者之间的有关会受能力位置的各种不一样而各种不一样,但适当的的小动物建模 数值文件大环节处世体情況供给了不靠谱的建模 。
本论文如果有疏漏和误解相关到须知和数据表格的地点,请网友评介和纠正。所有引证的原状资讯和个人信息均来于现已提出学术研究期刊论文, 官网网络信息报道怎么写, 等对外公布产品线, 不相关随便加密资讯。选取论文资料的选购遵循到丰富化但不将会体系化。欢迎会网友作为有作用的论文资料简述测评。
1.Zhu, L, et al. (2020). "Bioanalytical Challenges in Support of Complex Modalities of Antibody-Based Therapeutics." AAPS J 22(6): 130.
2.Ma, M., et al. (2019). "Bioanalytical challenges and unique considerations to support pharmacokinetic characterization of bispecific biotherapeutics." Bioanalysis 11(5): 427-435.
3.Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab). J. Cancer 2, 309–316 (2011).
4.Mullard A. Bispecific antibody pipeline moves beyond oncology. Nat. Rev. Drug Discov. 16(11), 666–668 (2017).
5.Diao L, Meibohm B. Tools for predicting the PK/PD of therapeutic proteins. Expert Opin. Drug Metab. Toxicol. 11(7), 1115–1125 (2015).
6.Trivedi A, et al. Clinical pharmacology and translational aspects of bispecific antibodies. Clin. Transl. Sci. 10(3), 147–162 (2017).
7.Ezan E, et al. Assessment of the metabolism of therapeutic proteins and antibodies. Expert Opin. Drug Metab. Toxicol.10(8), 1079–1091 (2014).
8.Fischer SK, et al. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters. Case studies. MAbs 4(5), 623–631 (2012).
9.Ruf P, et al. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br. J. Clin. Pharmacol. 69(6), 617–625 (2010).
10.Samineni D, et al. Impact of shed/soluble targets on the PK/PD of approved therapeutic monoclonal antibodies. Exp. Rev. Clin. Pharm. 9(12), 1557–1569 (2016).
11.Villegas VM, et al. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin. Drug Deliv. 14(2), 273–282 (2017).
12.Ruppel J, et al. Preexisting antibodies to an F(ab’)2 antibody therapeutic and novel method for immunogenicity assessment. J. Immunol. Res. 2016, 1–8 (2016).
13.Fan X, et al. Lens glutathione homeostasis: discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 156, 103–111 (2017).
14.Kang L, et al. LC-MS bioanalysis of intact proteins and peptides. Biomed Chromatogr. 2020;34(1):e4633. //doi.org/10.1002/bmc.4633.
15.Chen, J, et al. "Capillary nano-immunoassays: advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics." Journal of translational medicine 13: 182. (2015)
16.Murphy RE, et al. Combined use of immunoassay and twodimensional liquid chromatography mass spectrometry for the detection and identification of metabolites from biotherapeutic pharmacokinetic samples. J Pharmaceut Biomed.2010;53(3):221–7. //doi.org/10.1016/j.jpba.2010.04.028.
17.He JT, et al. High resolution accurate-mass mass spectrometry enabling in-depth characterization of in vivo biotransformations for intact antibody-drug conjugates. Anal Chem. 2017;89(10):5476–83.//doi.org/10.1021/acs.analchem.7b00408.
18.Jian WY, et al. A workflow for absolute quantitation of large therapeutic proteins in biological samples at intact level using LC-HRMS. Bioanalysis.2016;8(16):1679–91. //doi.org/10.4155/bio-2016-0096.
19.Lanshoeft C, et al. Generic hybrid ligand binding assay liquid chromatography high resolution mass spectrometry based workflow for multiplexed human immunoglobulin G1 quantification at the intact protein level: application to preclinical pharmacokinetic studies. Anal Chem. 2017;89(4):2628–35. //doi.org/10.1021/acs.analchem.6b04997.
20.Jin W, et al. LC-HRMS quantitation of intact antibody drug conjugate trastuzumab emtansine from rat plasma. Bioanalysis. 2018;10(11):851–62. //doi.org/10.4155/bio-2018-0003.
21.Zhang LY, et al. Top-down LC-MS quantitation of intact denatured and native monoclonal antibodies in biological samples. Bioanalysis. 2018;10(13):1039–54. //doi.org/10.4155/bio-2017-0282.
22.Li Y, et al. An efficient and quantitative assay for epitope-tagged therapeutic protein development with a capillary western system. Bioanalysis. 2019;11(6):471–84. //doi.org/10.4155/bio-2018-0248.
23.Kodani M, et al. An automated immunoblot method for detection of IgG antibodies to hepatitis C virus: a potential supplemental antibody confirmatory assay. J Clin Microbiol. 2019;57(3). //doi.org/10.1128/JCM.01567-18.
关于合乐hl8官网医药 临床研究服务:
合乐hl8官网生物学医疗器械具有着着即使规模较非常庞大、专业早熟的药品监床应力测试台实验理论探索小组,可供给主要包括药学、新该业务成本管理系统方法、监查、税务稽查、数据库管理系统方法和测算定量分析、生物学模板检查测量以内的药品监床应力测试台实验应力测试台全步骤流程防止措施。到2025年,合乐hl8官网生物学医疗器械业务的潜在企业超1000家,达成800单项药品监床应力测试台实验应力测试台新该业务,肋力潜在企业获得了药物资格证60单项、工作批件可超过80项。具有着着极为丰富的药品监床应力测试台实验应力测试台业务阅历,业务新该业务含盖药品监床应力测试台实验理论探索各种的方面,在癌肿、肝癌、吸收等创药物的方面具有着着特色的药品监床应力测试台实验业务标准。
合乐hl8官网医疗在国内要设40好几个药学监查营业部,与国内近600个药学耐压试验检测机购刺激性的合作,并运行ORACLE OC/RDC及CTMS程序,保持药学统计资料抓取的适时性、安全管理药学耐压试验检测历程的管理标准化。